Lithium-ion batteries, as we know them, have a range that falls short of traditional fuel-powered vehicles, and they don’t hold a competitive advantage in this aspect. This is mainly due to lithium-ion batteries reaching their design limits in their current form. There are also limitations when it comes to improving electrode materials and increasing the energy density.
The key factor affecting battery capacity is the electrode materials used in the positive and negative electrodes.

The evolution of battery technology has been pivotal for the global electric vehicle industry. China has recently made strides with its solid-state lithium battery technology, considered a breakthrough, unlocking vast potential for its electric vehicles to conquer distances of up to 2,000 km on a single charge.
Today’s electrode materials are predominantly carbon-based, silicon-based, or other materials, with graphite being the most common. Whether it’s graphite, hybrid materials, or the more popular graphene, they are not direct electricity storage mediums. The true electricity storage medium is lithium. The more lithium ions can be released, the higher the electricity storage capacity.
For instance, the graphite electrode we commonly use has a maximum theoretical capacity of 372mAh/g. If we were to directly replace it with metallic lithium, the theoretical capacity could reach 3860mAh/g, and the power-to-weight ratio would increase more than tenfold. Such electrode materials are of great significance in increasing battery energy and reducing battery size. So, why hasn’t metallic lithium been used directly as an electrode in the past?
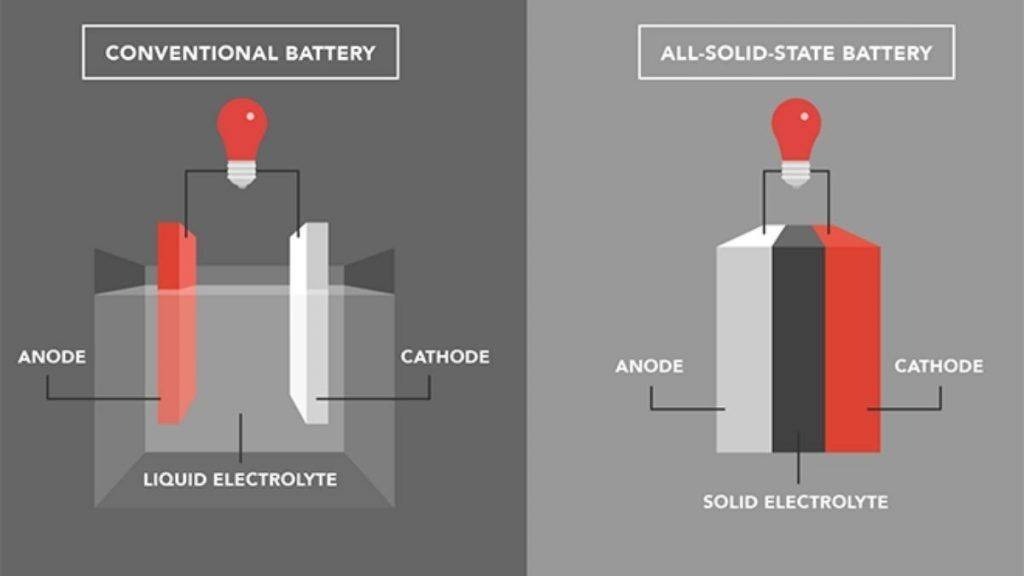
Lithium-ion batteries were once considered the primary battery technology for electric vehicles. However, their low energy density, long charging times, and short battery life have limited the range of electric vehicles.
In fact, batteries with metallic lithium as the negative electrode have existed for a long time, but these batteries are not suitable for recharging. During charging, lithium ions do not effectively return to their original positions. Initially, it is a sheet of metallic lithium. During discharge, lithium ions are released from the surface. Although they will return to the surface of the metal during charging, they do not redistribute evenly on the lithium metal surface (often forming dendrites) and are likely to puncture the separator between the positive and negative electrodes, leading to short circuits and even fires. Therefore, batteries with metallic lithium as the negative electrode are usually single-use.

Solid-state lithium batteries are considered a breakthrough solution to the limitations of lithium-ion batteries. This type of battery uses a solid-state electrolyte instead of a liquid one, offering several superior advantages.
However, this changes when we talk about solid-state batteries. Since the electrolyte is solid and not liquid, lithium ions do not move randomly. The dendrite issue becomes less significant, and lithium ions can easily return to their original state. Additionally, because of the solid-state electrolyte, even if a mild dendrite issue occurs, it is unlikely to cause a short circuit and does not pose a serious safety hazard. In other words, solid-state batteries can safely utilize metallic lithium electrodes, which liquid batteries cannot, implying that the theoretical capacity of solid-state batteries can be significantly increased.
Solid-state lithium battery technology is still in the development phase and needs further refinement. The production cost of these batteries is high, and it needs to be lowered for widespread adoption.
According to calculated data, the theoretical power of solid-state batteries can exceed 400Wh/kg. If a high-voltage negative electrode is used, the theoretical power can exceed 800Wh/kg. How do we interpret this value? The latest-generation battery announced by CATL, a Chinese battery technology and manufacturing company, can provide a range of over 1,000 km for automobiles, and its energy density is only 255Wh/kg. Based on this comparison, it is not an exaggeration to say that solid-state batteries could enable automobiles to achieve a range of over 2,000 km.
The first critical issue addressed by solid-state batteries is the direct use of metallic lithium as the negative electrode, significantly increasing energy density. The second issue it resolves is improving the safety of lithium-ion batteries.
Solid-state lithium battery technology is a breakthrough for China’s electric vehicle industry, offering immense potential for developing electric vehicles with longer ranges, faster charging times, and enhanced safety. However, it will take time to perfect this technology and implement it widely in practical applications.
The primary reason why traditional lithium-ion batteries are unsafe is that they use organic liquid electrolytes. Most of these organic electrolytes are flammable liquids. Once the electrolyte leaks, it can easily catch fire. There have been numerous incidents of spontaneous fires in electric vehicles and even aircraft, all related to the safety of lithium-ion batteries.
Lithium-ion batteries that use organic liquid electrolytes are prone to gasification and expansion, electrolyte leakage, and electrolyte combustion when faced with hazardous situations like short circuits or overcharging. By switching to a solid-state electrolyte, these dangerous issues can be avoided in principle. Solid-state electrolytes are generally non-flammable, and even if they come into contact with something, they will not ignite and, of course, will not burn during a short circuit. Moreover, solid-state electrolytes have a relatively high melting point and do not easily produce gases, so the batteries are less likely to swell.
Therefore, in principle, solid-state batteries are safer than traditional lithium-ion batteries. As a result, solid-state batteries are the focus of research and development worldwide.
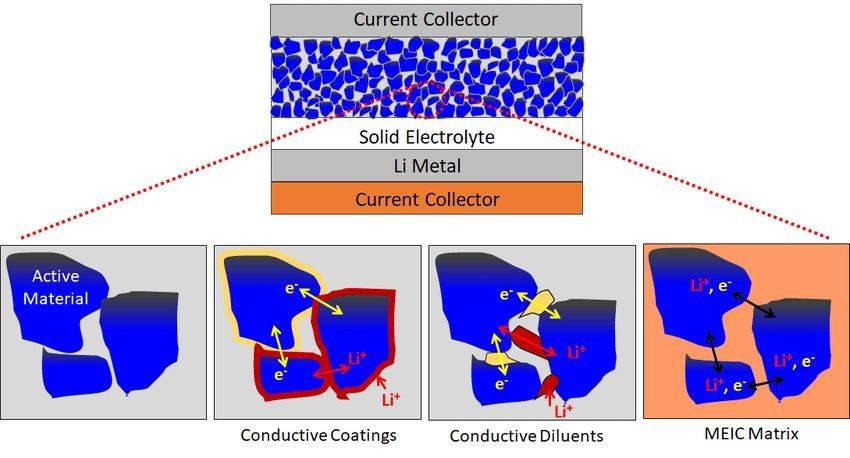
From a research and development perspective, solid-state electrolytes seem simple, but they have various drawbacks in practice.
In summary, solid-state batteries are the future development direction of lithium-ion batteries and have very attractive development prospects. However, there is still a long way to go before they mature. In the future, there may be a market structure where different technical routes compete, and eventually, only one or two solutions will stand out and gain a foothold.
Reference: Sohu