Food Safety Authority Recalls 18 Health Food Products Registered by Abbott Healthcare Vietnam
The Food Safety Authority (Ministry of Health) has just issued a document revoking the validity of the Registered Declaration of 18 health food products registered by Abbott Healthcare Vietnam Co., Ltd.
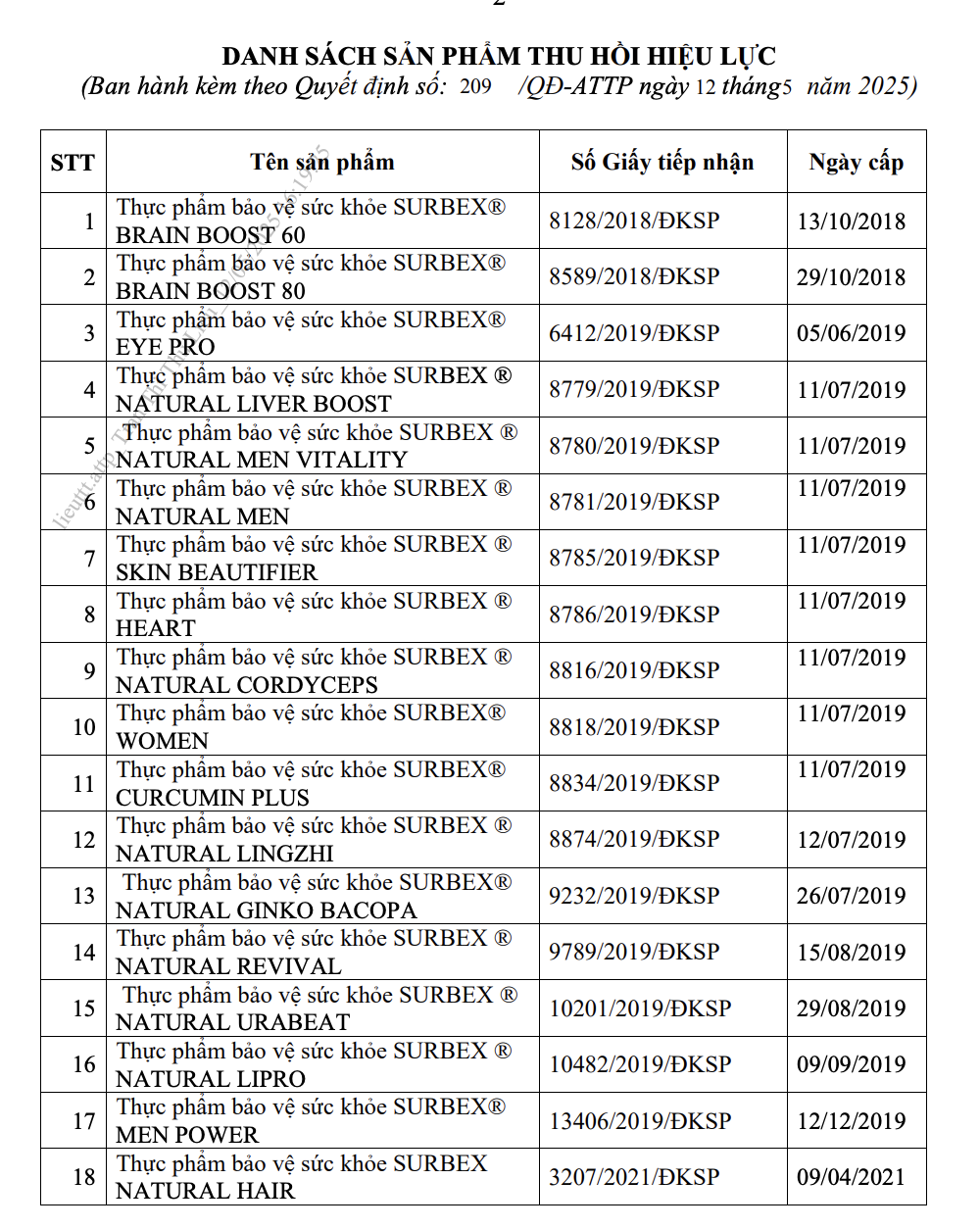
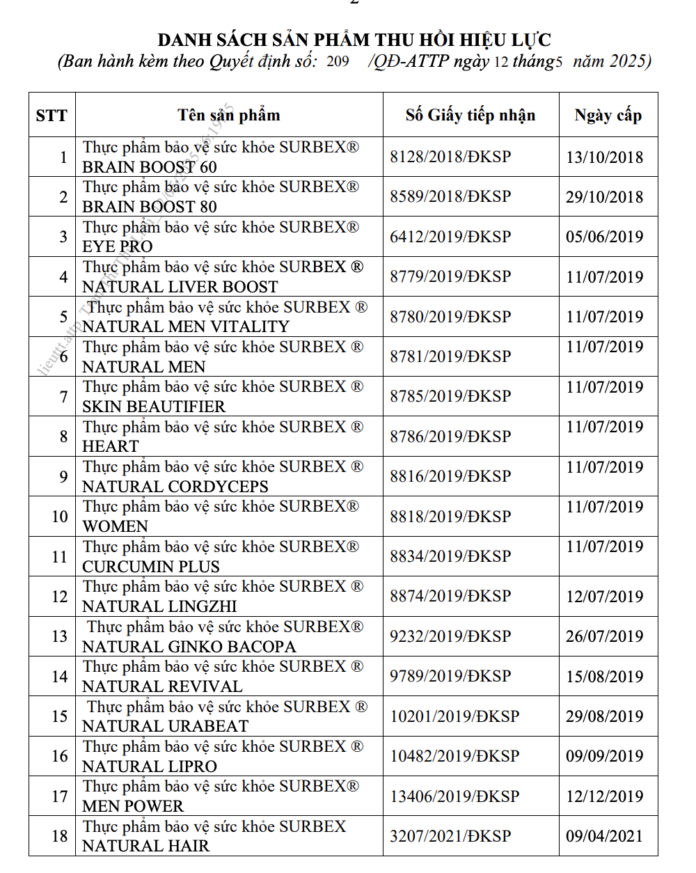
List of recalled products. Source: Food Safety Authority
Abbott Healthcare Vietnam Co., Ltd., formerly known as Glomed Pharmaceutical Co., Ltd., is located at 35 Tu Do Avenue, Vietnam-Singapore Industrial Park, An Phu Ward, Thuan An City, Binh Duong Province.
Among the 18 products whose registration declarations were revoked, there are health foods such as Surbrex Brain Boost 60 mg and 80 (brain-boosting pills), Surbrex Natural Liver Boost (heat-clearing and liver-detoxifying support), Surbex Natural Men Vitality (blood-tonifying and early hair graying risk reduction support), Surbex Natural Men (male reproductive health support), and Surbex Women (skin elasticity-enhancing and anti-aging support), etc.
Previously, Abbott Healthcare Vietnam Co., Ltd. had submitted a document requesting the withdrawal of the health food registration dossiers.
The Food Safety Authority requested the company and the Food Product Management Division and related departments to be responsible for executing this decision.




![[Photo Essay]: Experts, Managers, and Businesses Unite to Forge a Path Towards Sustainable Green Industry](https://xe.today/wp-content/uploads/2025/07/z678592918-150x150.jpg)


![[Photo Essay]: Experts, Managers, and Businesses Unite to Forge a Path Towards Sustainable Green Industry](https://xe.today/wp-content/uploads/2025/07/z678592918-100x70.jpg)