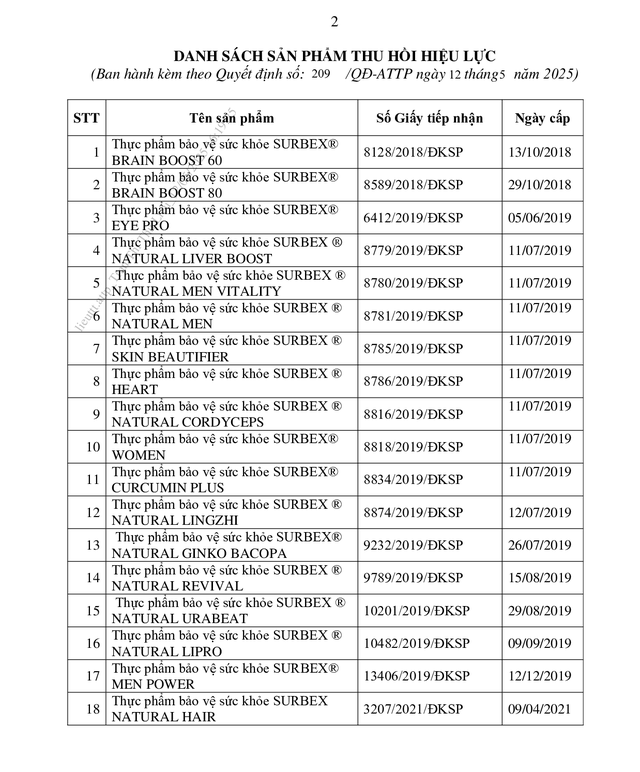
According to the Vietnam Food Administration (under the Ministry of Health), the agency has just issued a decision to revoke the Certificate of Registration for the announcement of 18 health protection product formulations announced by Abbott Healthcare Vietnam Co., Ltd. (formerly known as Glomed Pharmaceutical Co., Ltd.).
Abbott Healthcare Vietnam Co., Ltd., formerly known as Glomed Pharmaceutical Co., Ltd., is located at 35 Tu Do Avenue, Vietnam-Singapore Industrial Park, An Phu Ward, Thuan An City, Binh Duong Province.
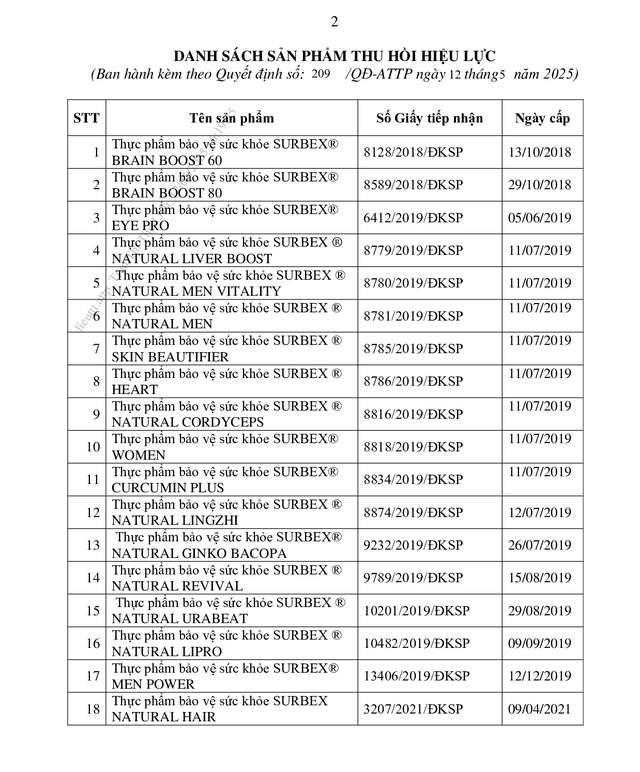
List of 18 functional foods whose registration announcement certificates were revoked.
Among the 18 products whose registration announcement certificates were revoked are Subrex Brain Boost 60 (brain-boosting pills), Subrex Natural Liver Boost (introduced as supporting heat reduction and liver detoxification), and Subrex® Skin Beautifier…
This decision takes effect from the date of signing and issuance (May 12) and replaces Decision No. 187/QD-ATTP dated April 29, 2025, of the Vietnam Food Administration on the revocation of the Certificate of Registration for the announcement of health protection products.
The Vietnam Food Administration requested that Abbott Vietnam Company, together with the Product Management Department and related departments, be responsible for executing this decision.
Recall of 18 Health Products by Abbott Healthcare Vietnam
The Ministry of Health has taken decisive action by revoking the authorization of 18 health-related products from Abbott Healthcare Vietnam Co., Ltd. This bold step underscores the Ministry’s unwavering commitment to safeguarding public health and ensuring that only safe and properly authorized products remain on the market.