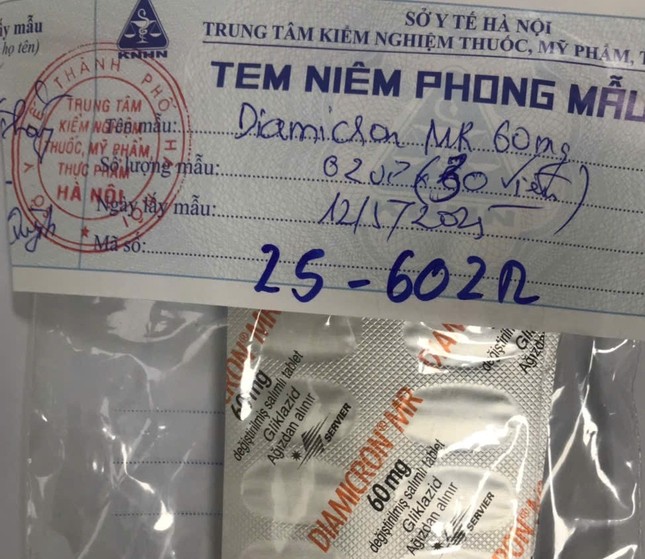
Hanoi’s Drug, Cosmetic and Food Testing Center reported that a batch of DIAMICRON® MR 60mg (Gliclazide) tablets, lot number 23F603, and expiration date of 04/2026, obtained from Duc Anh Pharmacy, failed to meet quality standards in terms of active ingredient quantification. Specifically, the analysis revealed that each tablet contained only 42.5 mg of Gliclazide, equivalent to 70.83% of the labeled content—significantly lower than the minimum allowed by the Vietnamese Pharmacopoeia V.
Additionally, six other batches of medication obtained from the same pharmacy lacked information on the Circulation Registration Number and/or Import License, as well as details about the manufacturing and importing entities.
The list of medications includes:
1. DIAMICRON® MR 60mg (Gliclazide), lot number: 23F603, expiration: 04/2026
2. Oseltamivir, lot number: M1164B01, manufactured: 03/2021, expiration: 03/2023
3. Crestor 20mg (Rosuvastatin), lot number: A23237030, expiration: 04/2026
4. Janumet 50/1000mg (Sitagliptin/Metformin), lot number: 24497505A, expiration: 07/2026
5. Plavix (Clopidogrel), lot number: ELB04027, expiration: 05/2027
6. NEXIUM® 40mg (Esomeprazole), lot number: 23H420, expiration: 09/2027
7. Crestor 10mg (Rosuvastatin), lot number: A24236004, expiration: 07/2027
Notably, many of these medications are original patented drugs, with high prices and indications for the treatment of cardiovascular diseases, diabetes, and dyslipidemia, raising concerns about the circulation of counterfeit and substandard drugs.
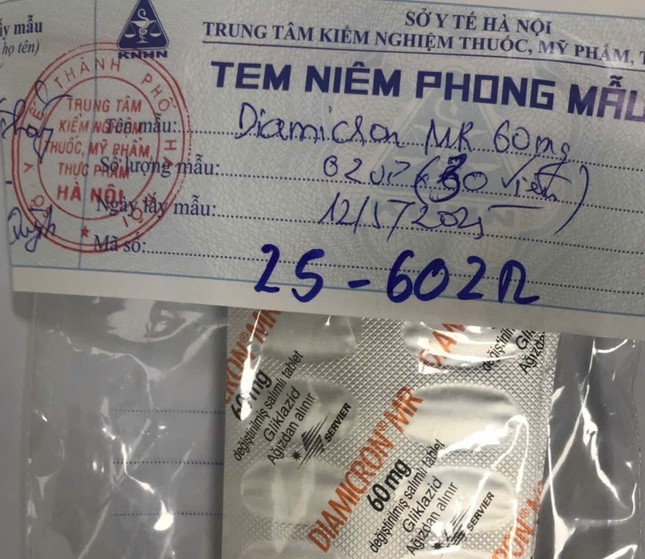
Substandard products are sealed by the Health Department.
Urgent investigation and traceability of drug sources:
In response to this situation, the Drug Administration Department has requested the Hanoi Department of Health to promptly report to the 389 Steering Committee and coordinate with the police, market management, and relevant authorities to conduct a comprehensive inspection of Duc Anh Pharmacy. They are also tasked with tracing the sources of the aforementioned batches of medication and strictly handling any violations in accordance with the law. A report on the results should be submitted to the Department before June 2, 2025.
Simultaneously, the Department has proposed that the Health Departments of provinces and cities strengthen communication to discourage both the public and medical facilities from purchasing and utilizing products without clear origins or lacking registration numbers or import licenses.
The public is advised to buy medicines only from licensed establishments and to immediately report any suspected counterfeit drugs to the health authorities and the police.
Furthermore, the local administration is urged to organize an intensive month of anti-counterfeit drug campaigns and rigorously implement the directives of the Government, the Ministry of Health, and the Drug Administration Department in this regard.
According to medical experts, a single tablet with an active ingredient content nearly 30% lower than the standard, as in the case of DIAMICRON MR, poses a significant danger to diabetic patients, potentially leading to treatment failure and increased risks of cardiovascular complications and renal damage.
This incident serves as a stark reminder of the loopholes in drug quality monitoring and the supply chain management in the market. As the pharmaceutical market recovers from the COVID-19 pandemic, tighter controls on drug sources and quality are more crucial than ever.