On May 29, 2025, the Drug Administration Department (Ministry of Health) issued Decision No. 278/QD-QLD on the recall of 14 cosmetic product declaration receipts voluntarily requested by Human Offshore Partners Software Services Co., Ltd. (formerly known as PH Vietnam Commercial Trading Co., Ltd.)
According to the Decision, the recalled products were once registered by the above-mentioned enterprise – the organization responsible for putting the products on the market – located at V1-A02 house, TTDV 01 land lot, new urban area An Hung, Duong Noi ward, Ha Dong district, Hanoi, enterprise code 0110018306.
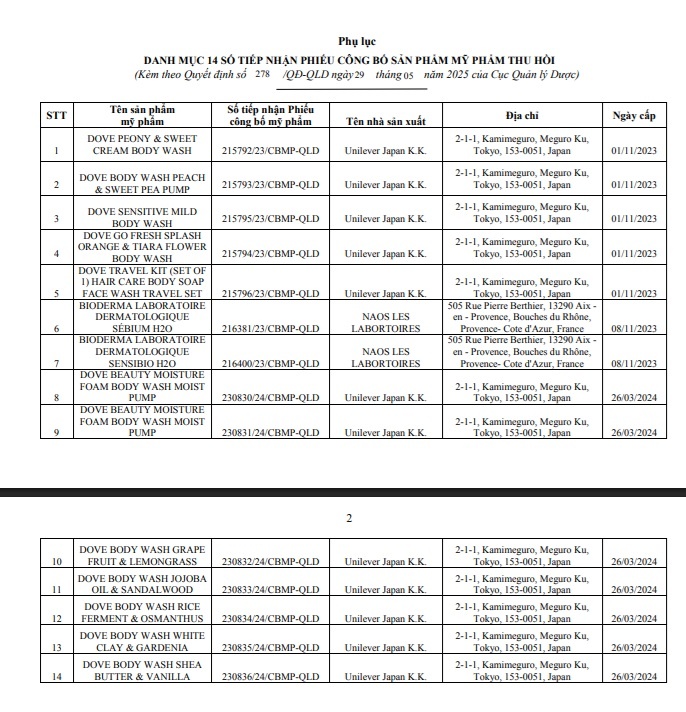
The list of products withdrawn from the management system includes some familiar cosmetics, notably Dove Peony & Sweet Cream Body Wash, receipt number 215792/23/CBMP-QLD issued on November 1, 2023. The product is manufactured by Unilever Japan K.K. and Bioderma Sebium H2O Micellar Water, receipt number 216381/23/CBMP-QLD issued on November 8, 2023, produced by NAOS LES LABORATOIRES (France).
According to current regulations, the receipt number of the cosmetic product declaration is granted by a competent state agency when an organization or individual declares that the product will be circulated in the market. However, this number does not confirm the product’s safety or effectiveness according to ASEAN standards. Each declaration receipt is valid for five years from the date of issuance and can be revoked in case of violation or voluntary request from the enterprise.
The Drug Administration Department stated that this recall is based on the voluntary request of Human Offshore Partners Software Services Co., Ltd. The Director of the company and the Director of the Department of Health of the provinces and centrally-run cities shall be responsible for executing this Decision.