On February 24th, the Drug Administration Department (Ministry of Health) announced the nationwide recall of the Peeling acne serum products from Bitechpharm biotechnology company and the E-Cosmetic Face wash gel products from LÁP Vietnam pharmaceutical company, distributed by Long Phụng Corporation. All of these companies are based in Ho Chi Minh City.
The reason for the recall is the use of ingredients not found in the cosmetic product’s registration file.
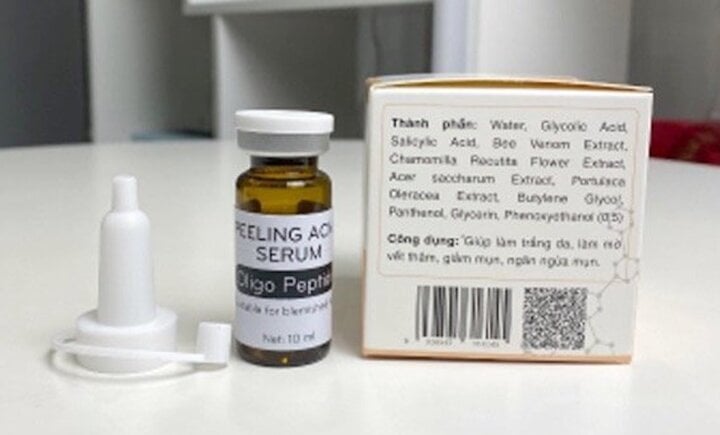
The Peeling acne serum product produced by Bitechpharm company.
The Drug Administration Department recommends the Departments of Health in provinces and centrally-run cities to announce to businesses and users of cosmetics in their areas to suspend trading the Peeling acne serum and E-Cosmetic Face wash gel products, and return the products to the suppliers.
Bitechpharm biotechnology company, LÁP Vietnam pharmaceutical company, and Long Phụng Corporation have sent recall notices to the distributors and users of the mentioned products.
At the same time, they will receive the returned products from businesses, proceed with the recall, and completely destroy the batch of cosmetics that do not meet the regulations. They will submit a report to the Drug Administration Department before March 31st.
The department recommends the Ho Chi Minh City Department of Health to recall the receipt numbers of the cosmetic product registration certificates for the two mentioned batches of cosmetics. They will monitor the companies’ implementation of the recall and destruction of the Peeling acne serum and E-Cosmetic Face wash gel products and provide a report before April 15th.