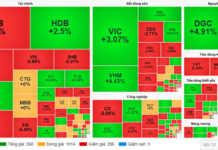
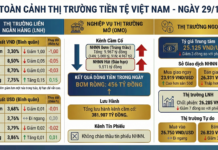
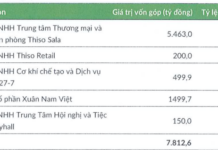
Services

Mr. Huynh Van Nhung, Vice President of Imexpharm, receives the Vietnamese Medicine Star Award
|
The Vietnamese Medicine Star Award for 2024 was organized by the Management Authority of the Ministry of Health based on strict and transparent criteria issued by the Ministry of Health in October 2023.
“Imexpharm is proud and honored to receive this prestigious and reputable award from the Ministry of Health and the Drug Administration,” said People’s Teacher, Pharmacist Tran Thi Dao, General Director of Imexpharm. “This is a testament to Imexpharm’s unwavering efforts in continuously investing in the quality of our factories and products for almost half a century. The award also affirms Imexpharm’s leading position in product quality and our role in providing good, effective, and affordable medicines to meet the healthcare needs of the people and medical facilities in Vietnam.

|
“Since 1977, Imexpharm has prioritized our strategy of investing in quality, research, and development,” shared People’s Teacher, Pharmacist Tran Thi Dao with pride. “We have consistently invested in our factories and production lines, and today, Imexpharm has become a leading pharmaceutical company in Vietnam, owning the largest EU-GMP factory cluster in the country with 3 EU-GMP factories and 11 EU-GMP production lines.”
Committed to this strategy, Imexpharm allocated 5% of its revenue in 2023 to R&D activities. As a result, the company has undertaken 91 research and development projects, with 15 already launched in the market, giving Imexpharm a competitive edge in new pharmaceutical products. In addition to investing in R&D, Imexpharm has expanded its EU MA license portfolio, initially identifying 30 potential target products. Last year, the company registered 11 additional EU MAs, bringing the total number of EU MAs it owns to 27 for 11 products. To promote innovative and generic drugs for the treatment of chronic diseases and reduce the financial burden on patients, Imexpharm has strengthened international cooperation to transfer technology for the production of generic and innovative drugs. In February, Imexpharm partnered with Genuone Sciences Inc., a leading South Korean pharmaceutical company, to transfer technology for the production of drugs for diabetes, cardiovascular diseases, and more.

|
In a challenging business environment and with changing disease patterns, antibiotics remain the dominant product line in the pharmaceutical market. Imexpharm continues to leverage its traditional strengths in this field, maximizing the production of antibiotics in its EU-GMP-certified factories.
Additionally, current health challenges such as environmental pollution, food safety issues, and work-related stress are driving the demand for respiratory, digestive, cardiovascular, and diabetes medications. With this in mind, Imexpharm is planning to expand its Non-Antibiotic product portfolio and is expected to build a new IMP5 factory in the Quang Khanh Industrial Park, Dong Thap. This new product line will include drugs for cardiovascular, diabetes, ear-nose-throat, cough, and digestive conditions, targeting not only the domestic market but also export opportunities. The company plans to receive the industrial land and commence construction in the second quarter of 2024. The factory is expected to be completed and operational by 2026-2027.
These strategies lay a solid foundation for the company’s vision towards 2030, with expectations to reach half a billion USD in revenue, marking a significant step in solidifying Imexpharm’s global position and meeting the growing demand for high-quality treatment solutions in Vietnam and the region.
|
About Imexpharm:
Founded in 1977 in Dong Thap, Vietnam, Imexpharm has always prioritized quality to provide safe, effective, and high-quality healthcare products for patients and the community. Today, Imexpharm owns four factory clusters, including three EU-GMP factory clusters with 11 production lines that meet EU-GMP standards. This makes Imexpharm the leading pharmaceutical manufacturer in Vietnam regarding EU-GMP factory clusters and the number of EU-GMP production lines. Imexpharm is the production partner of many leading multinational pharmaceutical corporations, including Sandoz, DP Pharma, Galien, Pharmacience Canada, and Sanofi – Aventis. As of 2023, Imexpharm has obtained 27 registrations for 11 products in Europe. For more information, please visit: https://imexpharm.com |