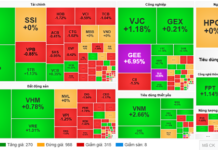
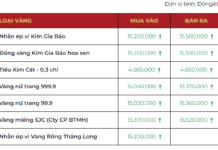
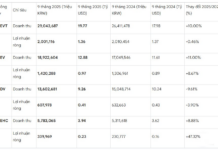
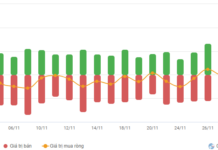
|
Nadyphar Company |
Nadyphar was fined VND 15 million for purchasing and selling Cetirizine (Cetirizine Hydrochloride 10mg) of substandard quality, a violation at Level 3 according to Vietnamese law. The Department of Health Inspection demanded that Mr. Huynh Nguyen Thanh, Nadyphar’s representative, comply with the administrative fine, and the company must strictly adhere to the decision.
Similarly, Nam Linh Pharmaceutical and Chemical Co., Ltd. was also fined VND 15 million for purchasing and selling Temozolomide Ribosepharm 100 mg, which violated Level 3 regulations.
The Department of Health Inspection imposed a fine of VND 50 million on Nam Viet Pharmaceutical Company for producing Carbomango medication after its registration for drug circulation had expired.
Additionally, the Drug Administration Department (Ministry of Health) sent a document to the Departments of Health of provinces and centrally-run cities regarding the suspension of circulation, recall, and destruction of cosmetics with ingredients listed on the label that differ from those declared on the product registration form of Kavr Import-Export Company Limited.
Specifically, eight cosmetic products were recalled and their circulation suspended nationwide: Roja Parfums Amber Aoud Parfum, Roja Parfums Diaghilev Parfum, Histoires De Parfums Ceci N’est Pas Un Flacon Bleu – This Is Not A Blue Bottle 1/.6 Eau De Parfum, Histoires de Parfums 1740 Eau de Parfum, Histoires de Parfums 1804 Eau de Parfum, Liquides Imaginaires Navis Eau De Parfum, Liquides Imaginaires Bello Rabelo Eau de Parfum, and Liquides Imaginaires Ile Pourpre Eau de Parfum. The reason for the recall is that the product formulas listed on the labels do not match the ones declared on the product registration forms.
Tung Phong