On September 16th, the Drug Administration of Vietnam reported that Novartis Vietnam received four user complaints regarding suspected counterfeit eye drops, including Tobrex, Maxitrol, and TobraDex. Upon investigation, batch VEE90A of Tobrex 5 ml was confirmed as counterfeit. Three other batches—Tobrex 5 ml (batch VEE98C), Maxitrol 5 ml (batch VFD09A), and TobraDex 5 ml (batch VHN07A)—are also under suspicion. These products were found circulating outside the company’s official distribution network.

Document from the Drug Administration of Vietnam – Image: Drug Administration of Vietnam
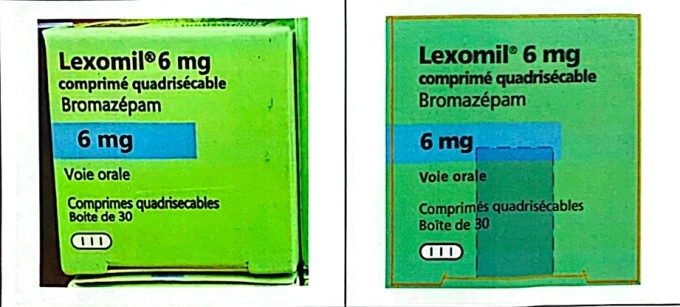
In another case, high-dose Lexomil 6 mg sleeping pills, owned by Cheplapharm Arzneimittel GmbH, were seized by Ho Chi Minh City Police. Batch F3193F01, with an expiration date of December 2027, was confirmed as counterfeit by the manufacturer. This batch was originally produced for the French market and was not imported into Vietnam. Lexomil 6 mg is not registered for circulation in Vietnam, making its sale and use illegal.
Lastly, Aclasta (containing zoledronic acid), used to treat osteoporosis, was flagged by a pharmacy in Kien Giang. The product, labeled with a production date of August 2024 and an expiration date of July 2027, was deemed suspicious. The registered company confirmed that this batch was not imported through official channels and noted irregularities in the packaging. Genuine Aclasta produced after May 2024 features the Sandoz logo instead of the Novartis logo. Products manufactured after this date with the old logo are suspected to be counterfeit.
Comparison of counterfeit (left) and genuine (right) medication provided by the manufacturer – Image: Drug Administration of Vietnam
In response, the Drug Administration of Vietnam has urged Health Departments across provinces and cities to urgently inspect, monitor, and notify pharmacies, hospitals, and the public.
The agency also calls for increased public awareness, encouraging citizens to purchase medications only from authorized outlets and avoid products of unknown origin. Companies such as DKSH, Sandoz, and Novartis are required to provide accurate information and cooperate in tracing the origins of these batches.
Additionally, the Administration reiterates the need to strictly enforce Directive 13/CT-TTg issued by the Prime Minister on combating smuggling, trade fraud, and counterfeit goods, along with Plan 614/KH-BYT issued in May 2025 to prevent counterfeit medications.
Amid rising counterfeit drug concerns, authorities advise the public to carefully verify packaging information against the Drug Administration’s registered database and promptly report any suspicious products to relevant agencies.
Raid on an illicit pharmacy uncovers a massive counterfeit diabetes and blood pressure medication ring; $7.8 million worth of fake drugs seized.
The Chinese authorities raided a pharmacy, seizing drugs advertised as a ‘panacea’ with blood lipid-lowering and blood pressure-reducing properties.
“The Conundrum of Candy: A Challenging and Costly Endeavor, Says Deputy Health Minister.”
“In a recent statement, Deputy Minister of Health Do Xuan Tuyen highlighted several cases of counterfeit medicine and food scandals in Vietnam. One notable example was a fake medicine ring in Thanh Hoa province, which was uncovered by the Ministry of Health and subsequently dealt with in collaboration with relevant authorities. Another incident involved Kera candies, which, according to Deputy Minister Tuyen, required extensive and costly testing to ensure public safety.”
Is It Legal to Accept Deposits Before Completing a Project’s Foundation Work?
“Despite its early construction stage, the project has already attracted buyers willing to put down deposits. This raises a valid question among investors: Is this legal? A thorough understanding of the applicable laws is crucial for all involved parties.”