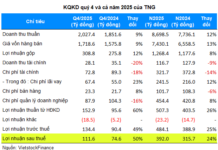
The Drug Administration of Vietnam (Ministry of Health) has issued Official Dispatch No. 2867/QLD-MP dated October 6, 2025, regarding the suspension and recall of three cosmetic products manufactured by Vinamake Joint Stock Company.
Specifically, the following three products are suspended and recalled nationwide:
1. DR. Hair Ginger Shampoo (Vinamake Brand), Registration Number: 24/24/CBMP-HB;
2. DR. Ginger Shampoo (Vinamake Brand), Registration Number: 23/24/CBMP-HB;
3. Nano Silver Feminine Wash with Floral Fragrance (Vinamake Brand), Registration Number: 122/23/CBMP-HB.

Image source: thuonghieucongluan.com.vn
These products are manufactured and distributed by Vinamake Joint Stock Company, located at Group 8, Le Thanh Tong Street, Tan Thinh Ward, Hoa Binh City, Hoa Binh Province (now Group 8, Le Thanh Tong Street, Hoa Binh Ward, Phu Tho Province).
The recall is due to product samples tested by the Institute of Criminal Science under the Ministry of Public Security, which found discrepancies between the declared ingredients and those listed on the product labels and packaging. The Economic Police Department of Phu Tho Provincial Police concluded that these products are “counterfeit” under Article 3, Clause 7(b) of Decree No. 98/2020/NĐ-CP dated August 26, 2020, issued by the Government.
The Drug Administration urges the Departments of Health in all provinces and cities to enhance communication and inform all cosmetic retailers, users, and the public to cease trading and using the aforementioned products. Retailers should return the products to suppliers and promptly report any findings to the relevant authorities. Additionally, local authorities are instructed to inspect and supervise the recall process, taking action against violators as per current regulations.

Illustrative image
The Departments of Health are also directed to instruct Testing Centers to increase sampling and quality checks of cosmetics in their jurisdictions. A hotline should be established to receive reports on counterfeit, smuggled, or untraceable cosmetics, ensuring timely verification and handling in collaboration with relevant agencies.
Vinamake Joint Stock Company is required to notify all distributors and users of the affected products, accept returns from retailers, and submit a recall report to the Drug Administration by November 1, 2025.
The Drug Administration requests the Department of Health of Phu Tho Province to collaborate with local authorities in overseeing Vinamake’s recall process. A report on the inspection and supervision results must be submitted to the Drug Administration by November 15, 2025.
Urgent Health Ministry Alert: List of Eye Drops and Osteoporosis Medications Suspected of Counterfeiting
On September 16th, Vietnam’s Drug Administration (under the Ministry of Health) issued an urgent warning regarding three groups of drugs suspected of being counterfeit. This alert followed reports from pharmaceutical companies and consumers.