Outstanding Financial Results
On October 20, 2025, Imexpharm Joint Stock Company announced its Q3 and 9-month business results for 2025. Imexpharm continued its positive growth across all key metrics, driven by stringent cost management and robust sales performance.
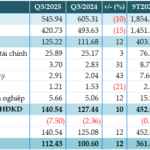
In Q3 2025, the company recorded a 10% increase in gross revenue and a 5% rise in net revenue compared to the same period last year, despite industry challenges. This reflects Imexpharm’s ability to sustain growth in both OTC and ETC channels.
Cost of goods sold increased only 3% year-over-year—lower than revenue growth—thanks to efficient production planning, optimized factory capacity, and cost-saving measures across four manufacturing clusters. In Q3, Imexpharm also launched the final lyophilized powder production line at its EU-GMP-certified IMP4 facility, further enhancing its EU-standard manufacturing capacity.

Research and development of EU-GMP-quality products at Imexpharm’s facility. Photo: Imexpharm.
|
As a result, gross profit rose 9% year-over-year, with a stable 40% gross margin.
Operating expenses remained tightly controlled, with selling and administrative costs increasing just 3% year-over-year, demonstrating disciplined spending amid market expansion and digital transformation investments.
With strong cost control and operational efficiency, net profit before tax (NPT) grew 11%, and EBITDA increased 12% year-over-year, highlighting the company’s focus on profitability.
Quarterly results dipped slightly compared to the high-growth Q2, primarily due to two temporary factors: increased Q2 purchases ahead of July price adjustments and policy changes effective July, including business tax regulations, provincial administrative mergers, and two-tier governance implementation.
After a slower August, sales rebounded strongly in September, with net revenue up 25% month-over-month, NPT surging 101%, and EBITDA rising 48%, confirming robust market demand.
For the first nine months of 2025, gross revenue reached VND 2,118 billion, up 21% year-over-year, and net revenue hit VND 1,800 billion, a 16% increase. This growth was driven by strong performance in both OTC (up 19%) and ETC (up 21%) channels.
Gross profit increased 21%, NPT rose 23%, and EBITDA grew 18% year-over-year. Gross margin remained at 40%, while EBITDA margin improved to 22.3%, reflecting balanced growth and operational efficiency.

Quality control line at Imexpharm’s EU-GMP-certified IMP4 facility. Photo: Imexpharm.
|
As of September, Imexpharm achieved 68% of its full-year net revenue target and 63% of its NPT goal, maintaining strong progress toward 2025 objectives.
In the first nine months, Imexpharm launched 20 new products, surpassing its annual plan of 16 SKUs, and continued innovation with 144 active R&D projects.
ESOP Share Issuance
Per the Board of Directors’ resolution on September 23, 2025, shareholders approved the issuance of over 1.55 million ESOP shares at VND 5,000 per share, with a one-year transfer restriction. As of September 30, 2025, IMP shares had risen 14% year-to-date.
Transitioning from cash bonuses to ESOP shares preserves cash flow while aligning employee and company long-term interests. Once approved, this program will strengthen workforce development and investor confidence in Imexpharm’s disciplined management and sustainable growth commitment.
Market Outlook
Digital transformation, AI integration, and the rise of specialty and biological drugs are reshaping global R&D. Amid these shifts, high-quality generics and biosimilars remain essential for managing rising healthcare costs.
 Imexpharm’s EU-GMP-certified IMP4 facility. Photo: Imexpharm.
|
Vietnam’s pharmaceutical industry is entering a new growth phase, supported by government initiatives to enhance domestic drug production and digital healthcare transformation. With its EU-GMP leadership and commitment to innovation, Imexpharm is well-positioned to contribute to Vietnam’s healthcare development.
People’s Doctor and Pharmacist Trần Thị Đào, Imexpharm’s CEO, stated: “The market is evolving with opportunities and challenges. Our focus on quality and adaptability underpins Imexpharm’s continued success. We enhance competitiveness through product innovation, market expansion, operational efficiency, and long-term growth investments.”
Services
– 06:00 23/10/2025
Novaland Defers Nearly VND 95 Billion in Bond Principal Repayments
Novaland has deferred nearly VND 94.6 billion in principal payments for its NVLH2123003 bond issuance due to insufficient payment arrangements.
Unveiling the Post-Restructuring Operational Model: How Coteccons Thrives as a Leaderless Corporation
At Coteccons, the operational model is structured around a functional organization led by the Senior Management Committee (SCOM), comprising seven members who collectively assume the responsibilities traditionally held by a CEO. This innovative approach is championed by Ms. Nguyễn Trình Thùy Trang, Deputy CEO of the Operations Division.