Recall of 162 Products Due to Missing PIF Documentation
On November 25th, the Drug Administration of Vietnam’s Ministry of Health officially issued a recall order for 162 cosmetic products and 80 registration numbers associated with MK Skincare, a subsidiary of the Mailisa ecosystem.
The reason for this action is the company’s failure to provide Product Information Files (PIF), a mandatory document in the cosmetics industry. PIFs are essential to verify product quality standards, manufacturing processes, and safety. Without PIFs, these products lack proof of quality, have unverifiable origins, and pose potential health risks to consumers.
Mailisa’s product lineup before the recall, with only 3 out of 100 flagship products generating billions in revenue for the company.
The recall is being executed in full compliance with legal regulations. Importantly, this is a mandatory recall ordered by state authorities, not a voluntary action by the company.
News of the recall quickly sparked widespread discussion on social media. Many consumers shared that they had spent millions to tens of millions of Vietnamese dong on Mailisa/MK Skincare skincare sets.
Numerous users reported incomplete treatments, with some experiencing skin irritation and redness. Most expressed concern over the refund and exchange process, as many Mailisa branches have suspended operations due to ongoing investigations.
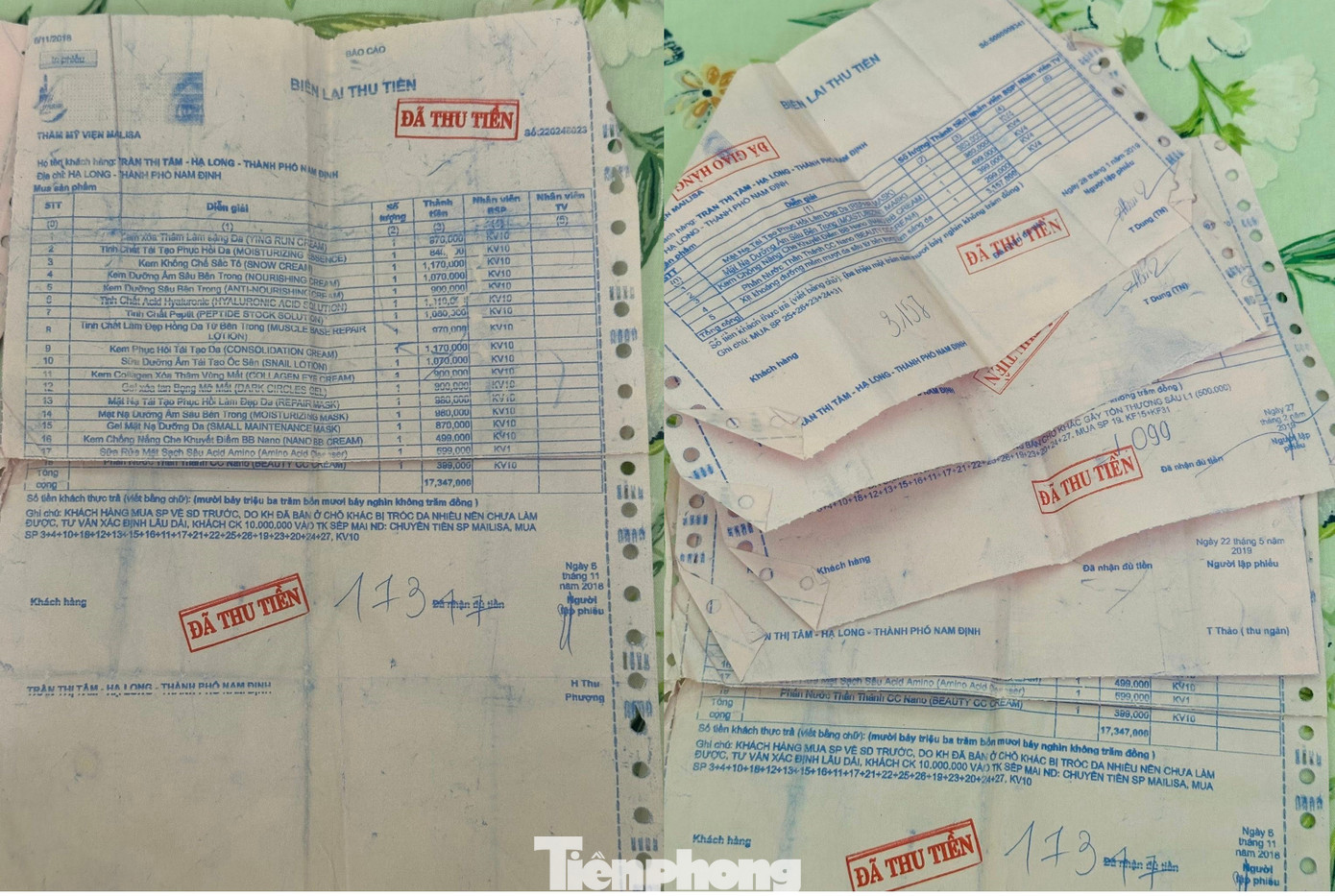
A customer’s receipt showing a purchase of tens of millions of dong worth of Mailisa products.
One customer lamented, “I spent over 17 million dong on a full set, only to find out now that the products lack safety documentation. Who do I turn to for a refund?”
Companies Must Refund and Compensate for Damages
According to Attorney Hạ Thị Thu Thảo (Ho Chi Minh City Bar Association), the Ministry of Health’s recall decision provides a clear legal basis for consumers to demand their rights, including refunds, product exchanges, or compensation for health-related damages.

Attorney Hạ Thị Thu Thảo.
Attorney Thảo emphasized, “When a mandatory recall is issued by regulatory authorities, companies are legally obligated to assume civil liability toward consumers. Even in cases of unintentional negligence, compensation is required if the products cause harm.”
Under the 2023 Consumer Protection Law and Decree 54/2024, all entities in the supply chain (manufacturers, importers, distributors, and retailers) must: Provide accurate and transparent product information; ensure quality and safety; recall defective products; publicly disclose recall reasons, collection points, and exchange procedures; and proactively compensate affected customers.
Mailisa’s promotional campaigns on social media.
Legal experts advise Mailisa/MK Skincare customers to take the following steps: Retain all related documents, including receipts, purchase messages, product photos, packaging, and labels, as evidence for refund claims. Immediately discontinue product use to avoid potential health risks. Submit refund/compensation requests to the seller in writing or via email, citing the Ministry of Health’s recall decision and the Consumer Protection Law.
If the company fails to respond, seek intervention from authorities such as the Drug Administration, the Competition and Consumer Protection Department, or the local Health Department where the product was purchased.
To protect the community, users are encouraged to share recall information to prevent further use of banned products.

Mailisa’s Ho Chi Minh City branch has ceased operations.
Attorney Hạ Thị Thu Thảo noted that this large-scale recall marks one of the most significant enforcement actions in Vietnam’s cosmetics industry in recent years. The company’s failure to provide PIF documentation and the subsequent mandatory recall expose critical gaps in product registration and distribution processes, underscoring the need for tighter market regulation. Amid ongoing investigations into Mailisa’s alleged smuggling activities, ensuring consumer refunds, compensation, and health protection has become an urgent priority.
Unveiling the Billion-Dollar Black Market: Mailisa’s Illegal Cosmetics Smuggling Ring from China Exposed
Mailisa, also known as Phan Thị Mai, and her husband, Hoàng Kim Khánh, have built a lucrative business by sourcing inexpensive cosmetics from China and smuggling them into Vietnam, generating profits in the billions of dong.
Mailisa Headquarters in Ho Chi Minh City Shuts Down Following CEO’s Indictment
On the morning of November 22nd, our reporter observed that Mailisa Beauty Clinic, located at 86-88-91 Huynh Van Banh Street, Cau Kieu Ward, Ho Chi Minh City, had temporarily ceased operations. This followed the arrest and prosecution of Ms. Phan Thi Mai, Director of Mailisa Beauty Clinic Co., Ltd., by the Ministry of Public Security’s Investigation Agency on charges of “smuggling.”
Unveiling Anomalies: 81 Mailisa Products Mysteriously Vanish from System Amidst Hundred-Billion Revenue Ecosystem
Unveiling a startling development, 81 Doctor Magic cosmetic products, once officially approved by the Ministry of Health and proudly featured on Mailisa’s website, have mysteriously vanished. These products are now under scrutiny by the Drug Administration Agency for quality assessment.