Illustrative Image
Regarding the official dispatch from the Drug Administration and information circulated across various media outlets, Diana Unicharm has issued an official statement to inform consumers.
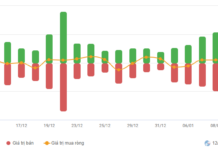
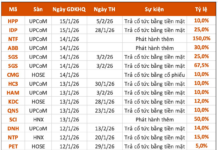
Diana Unicharm confirms that the batch of Fresh & Care Odor Fresh+ products subject to recall, due to the omission of one ingredient as per regulations, is entirely safe for consumer health. This batch is no longer available in the market.
Through internal inspections, Diana Unicharm identified that the Fresh & Care Odor Fresh+ product with batch number 24101101 and expiration date 11/10/2026 failed to list Sodium Benzoate (Natri Benzoate) as required. This oversight pertains solely to registration procedures and does not compromise the product’s quality or safety.
The company disclosed that upon discovering the issue in September 2025, it promptly initiated a full market recall of the affected batch and implemented necessary corrective measures. Diana Unicharm assures that currently available Fresh & Care Odor Fresh+ products fully comply with ingredient disclosure regulations.
Diana Unicharm is actively collaborating with relevant authorities to strictly adhere to the Drug Administration’s guidelines. The company remains committed to enhancing internal controls to deliver safe, high-quality products that support Vietnamese women.
*Sodium Benzoate (Natri Benzoate) is a widely used preservative in the food, cosmetic, and pharmaceutical industries, recognized for its safety and efficacy in inhibiting bacterial, yeast, and mold growth.
The Ultimate Showdown: Unveiling the An Phu Interchange Project
Facing the risk of delays that could extend until the end of 2026, the People’s Committee of Ho Chi Minh City has instructed the investor of the An Phu intersection project to complete and commission the construction before December 31 this year, as per the original schedule, with a resolute commitment to avoiding any further postponements.