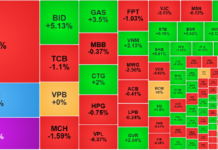
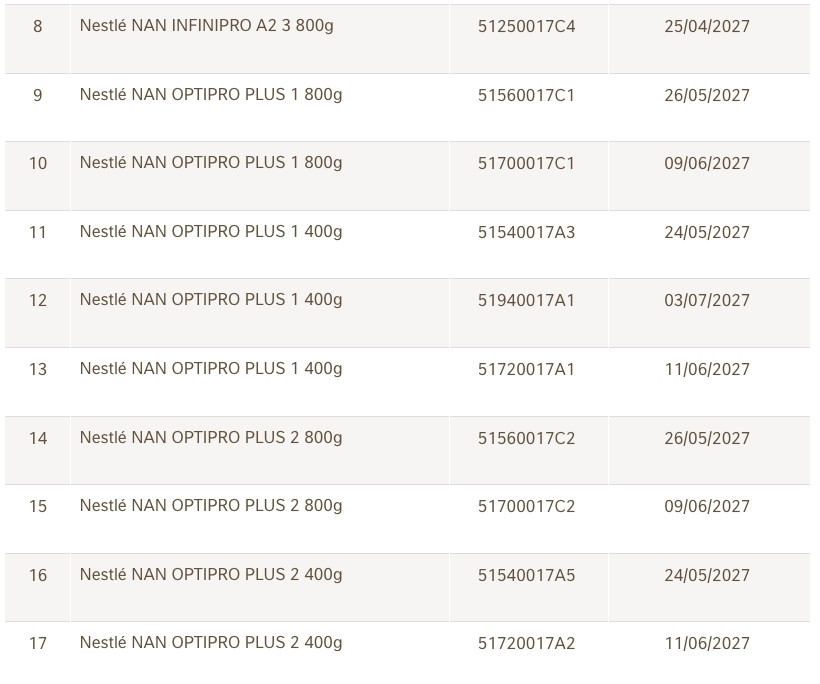
Nestlé Vietnam Co., Ltd. announces a voluntary and proactive recall of 17 batches of NAN infant formula imported and distributed in Vietnam. The specific batch numbers have been disclosed by the company in the accompanying list.

According to Nestlé Vietnam, this decision follows the corporation’s routine review of raw materials. Testing revealed that a raw material, PUFA oil supplied by a third-party European vendor, may contain traces of Cereulide—a toxin produced by Bacillus cereus bacteria. This ingredient is used in minimal quantities in certain infant formulas manufactured in Europe.
The company emphasizes that, to date, there is no evidence of Cereulide in the recalled NAN batches. However, adhering to the principle of utmost caution and ensuring consumer safety, Nestlé has opted to recall potentially affected batches as a preventive measure, aligned with its stringent quality management standards.
Nestlé Vietnam confirms that products not included in the 17 recalled batches—such as NAN OptiproPlus (Steps 3 & 4), NAN SupremePro (3 Steps), S-26 Ultima (3 Steps), PreNAN, NAN ExpertPro Lactose Free, NAN ExpertPro Total Comfort, Nutren Junior, Peptamen Junior, and other distributed products—remain safe and of assured quality for consumption.
This recall in Vietnam follows the Ministry of Health’s earlier directive to halt sales and remove information for certain Nestlé Beba and Alfamino batches due to bacterial toxin concerns. The Food Safety Department has urged authorities to intensify inspections and monitor the sale of these products, particularly on e-commerce platforms.
Previously, Nestlé initiated a recall of multiple infant formula batches across dozens of countries after identifying Cereulide risks in raw materials. The recall began in late 2025 and expanded in January 2026, following the completion of necessary safety tests.