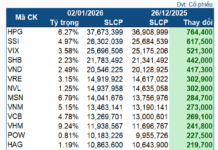
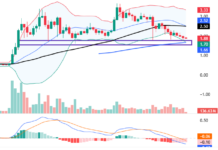
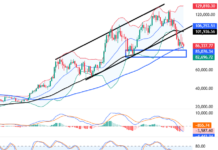
VN-Index Surges 1.81% to 1,640.69, Breaking Past the Psychological Barrier with Widespread Green. Market liquidity reached 58.4 trillion VND, the highest in many weeks, indicating optimism despite foreign investors’ net selling of over 2,400 billion VND. Capital flowed into banking and real estate, prioritizing stocks with strong fundamentals. Notably, SCR led the real estate group, surging 5.05% to 10,400 VND per share, surpassing the 52-week high, climbing 84.4% year-to-date, and 25.85% month-to-date. Impressive 6-month financial results with a profit of 50.69 billion VND, achieving 101.4% of the full-year plan, showcase the company’s robust performance.
This two-digit price marks a significant milestone for SCR, which has traded in a lower range for an extended period, even dipping below 5,000 VND per share at times. This development occurs amidst a buoyant broader market.
Favorable macro-economic conditions played a crucial role. Policy rates and deposit rates remained low, while credit was facilitated for viable projects, particularly in real estate, reducing capital costs and encouraging a shift in funds from savings to securities. With its unique characteristics, including financial leverage and strategically located land banks, TTC Land benefited doubly from this trend, reinforcing investors’ expectations of improved profit margins in the upcoming quarters.
However, SCR’s upward momentum stems from more than just market factors. In the first half of 2025, TTC Land demonstrated its project execution capabilities and expanded international cooperation with a series of significant milestones. In May, the company topped out and handed over the commercial block of the TTC Plaza Da Nang project to AeonMall Vietnam, simultaneously signing a hotel management agreement with TUI Hotels & Resorts, a renowned international tourism brand, for the TUI SUNEO hotel at the same location. In parallel, TTC Land introduced the “Dao Kim Quy” brand for its large-scale island resort and entertainment project, envisioned as a strategic highlight in the long term. Notably, the Q2 2025 financial report revealed a fivefold increase in revenue compared to the same period last year, with profits surpassing 101% of the full-year plan within just six months, reflecting robust sales and project development performance.
SCR’s resurgence serves as an early indicator of investors’ renewed faith in the sector’s comeback. This surge encapsulates three key factors: cheap money, a warming real estate market, and TTC Land’s preparedness to ride the new growth wave.
The confluence of positive macro-economic conditions and robust financial results has propelled SCR into the spotlight, attracting substantial market attention in recent sessions. Nevertheless, the 10,000 VND per share milestone is merely a starting point. To sustain long-term growth, TTC Land must continue to mitigate financial risks, ensure project progress and quality, and capitalize on opportunities presented by the real estate sector’s recovery.
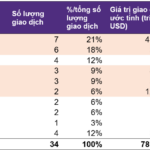
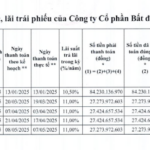
What is the Outstanding Bond Debt of Dragon Village?
In the first half of 2025, Dragon Village made interest payments totaling over VND 190 billion across five bond batches, from DVLCH2124001 to DVLCH2124005.
The Legend Danang: A Legend Unfurls by the Dragon Bridge
The Legend Danang emerges as a rare “iconic landmark” project, situated right next to the Dragon Bridge symbol. It embodies a unique dual value proposition: a cultural and tourism landmark that also offers robust potential for value appreciation, with Danang poised to become the financial and economic hub of the region in the future.
Over 1,200 Business “Warriors” Unite at the T&T City Millennia Kick-off Event
On August 14th, the T&T City Millennia project’s kick-off event in Ho Chi Minh City attracted over 1,200 real estate agents and strategic partners, showcasing the allure of this premium riverside urban development in southern Saigon. This event marks the beginning of T&T Group’s journey into the southern real estate market, building upon their successful large-scale projects across Vietnam.