Dengue Fever Vaccine by Takeda, Japan: A Revolutionary Solution for Vietnam
Takeda, a renowned Japanese pharmaceutical company, introduced the world’s first dengue fever vaccine in 2018. As of 2024, it has been approved for use in over 40 countries, with a particular focus on those facing complex dengue outbreaks.
Utilizing modern technology, the vaccine offers comprehensive protection against all four dengue virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4), with an impressive efficacy rate of over 80%. Additionally, it provides up to 90% protection against hospitalization due to dengue.
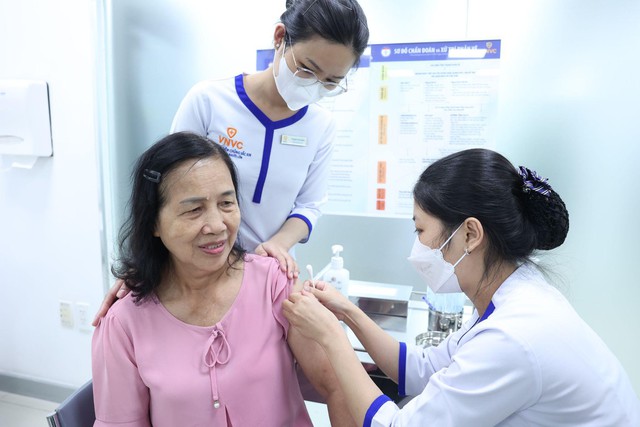
Individuals receiving the dengue vaccine at VNVC’s vaccination system.
Dengue fever is a devastating disease that has plagued numerous countries worldwide. In recent years, the epidemiology of dengue has changed, with cases increasing steadily each year instead of following a cyclical pattern. This shift is attributed to the consequences of urbanization and global warming, which have provided ideal conditions for the proliferation of disease-carrying mosquitoes.
All four dengue virus serotypes that cause dengue fever are currently circulating in Vietnam, with DEN-1 and DEN-2 being the most prevalent. DEN-2 is of particular concern as it is often associated with severe cases, outbreaks, and fatalities. Additionally, there has been a rise in the number of severe cases, possibly linked to the dominance of the DEN-2 virus, as well as an increase in patients with underlying health conditions and obesity.

VNVC’s GSP-compliant cold truck system, likened to a “mobile cold warehouse,” efficiently transports the dengue vaccine to vaccination centers for the benefit of the community.
Dr. Nguyen Minh Tuan, Head of the Dengue Fever Department at Children’s Hospital 1 in Ho Chi Minh City, stated that while dengue fever can affect both children and adults, children are at a higher risk of developing severe complications and have a higher mortality rate. Many children are hospitalized with severe complications, including shock, cardiovascular collapse, massive bleeding, and reduced circulatory volume, which pose a significant threat to their lives.
According to WHO data, the number of dengue cases has increased tenfold in the last two decades, from 500,000 cases in 2000 to over 5 million in 2019. In Vietnam, the situation has been dire, with two peak outbreaks in 2019 and 2022 within a short span of three years. In 2022 alone, the country recorded more than 367,000 cases, ranking second globally, just behind Brazil.
The aftermath of surviving severe dengue with complications is grim. Nearly 70% of patients experience reduced work capacity, and over 50% live with long-term symptoms such as joint and muscle pain, fatigue, weakness, hair loss, and more, for up to two years.
Currently, there is no specific treatment for dengue fever. Medical management focuses on supportive care, including blood transfusions, plasma exchange, and shock prevention. The financial burden of treating severe dengue can be significant, with costs running into the hundreds of millions of dong.
In May 2024, the Vietnamese Ministry of Health officially approved the use of Takeda’s dengue vaccine in the country.
Vu Thi Thu Ha, Director of Supply at VNVC, a comprehensive strategic partner of Takeda, shared that they had placed early orders and worked diligently with the manufacturer to secure a large quantity of the dengue vaccine for Vietnam.
“The introduction of the dengue vaccine in Vietnam will alleviate the disease burden on the population, reduce hospitalizations and complications, prevent healthcare system overload, and enable hospitals to allocate resources to treating non-communicable diseases,” said Dr. Tuan, expressing his optimism about the vaccine’s impact.

VNVC boasts international GSP-compliant cold storage and cold chain systems, capable of simultaneously storing up to 400 million doses of high-quality vaccines.
Over the past eight years, VNVC has collaborated with leading global vaccine manufacturers to introduce dozens of new vaccines to Vietnam, providing the community with access to critical vaccinations against various dangerous diseases. Since the beginning of 2024, VNVC has brought in three new vaccines: the latest generation of the meningitis B vaccine, the pneumococcal 23 vaccine, and the dengue vaccine. In the near future, VNVC plans to introduce the zona nerve vaccine to prevent new cases and recurrence in individuals aged 18 and older.
“Vingroup Signs MoU with Warner Music Group and Indochina Productions”
On September 25, 2024, Vingroup (HOSE: VIC) inked a memorandum of understanding with two powerhouses in the global music and film industries, Warner Music Group and Indochina Productions. This collaboration not only brings world-renowned music stars to Vietnam but also serves as a launchpad for the country’s tourism and cultural image to reach a global audience of hundreds of millions.










![VPBank Proudly Presents G-DRAGON 2025 WORLD TOUR [Übermensch] IN HANOI as Title Sponsor](https://xe.today/wp-content/uploads/2025/10/screen-sho-2-218x150.png)