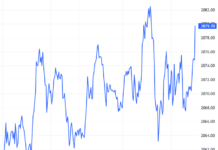
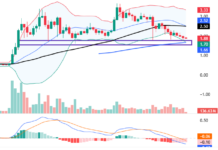
In May 2025, Vietnam’s pharmaceutical market witnessed one of its largest mergers and acquisitions (M&A) deals to date. China’s Livzon Pharmaceutical Group Inc., through its Singapore-based subsidiary Lian SGP Holding Pte. Ltd., signed an agreement to acquire approximately 64.81% of the shares in Imexpharm Pharmaceutical Joint Stock Company (stock code: IMP). The total value of the deal is estimated at over VND 5,730 billion (approximately USD 220.6 million).
According to the announcement, Lian SGP Holding Pte. Ltd. will acquire all Imexpharm shares from existing shareholders, including SK Investment Vina III Pte. Ltd. (owned by South Korea’s SK Group) with a 47.69% stake, Sunrise Kim Investment JSC with 9.75%, and KBA Investment JSC with 7.37%.

Later in 2025, Vietnam’s National Competition Commission (under the Ministry of Industry and Trade) issued a decision regarding this acquisition, imposing four conditions on Lian SGP Holding Pte. Ltd., Imexpharm, and their affiliated entities.
Lian SGP Holding Pte. Ltd. and its affiliated companies are prohibited from discriminating against Imexpharm or other Vietnamese enterprises in terms of commercial conditions when supplying antibiotic active pharmaceutical ingredients (APIs).
Lian SGP Holding Pte. Ltd. and its affiliates must maintain a stable production and business strategy, ensuring a consistent supply of high-quality antibiotics to Vietnamese consumers.
Within the year of completing the economic concentration transaction, Lian SGP Holding Pte. Ltd. and its affiliated entities are required to develop and submit a written report to the National Competition Commission outlining plans to enhance the positive impacts of the merger. This includes: a) Strengthening Imexpharm’s research and development (R&D) capabilities; b) Facilitating technology transfer for the production of advanced pharmaceuticals in alignment with Imexpharm’s manufacturing and distribution capacity.
A written report must be submitted every three years (by March 31st of the reporting year) starting from the completion of the transaction and upon request by the National Competition Commission to ensure compliance with the imposed conditions.
The divestment from Imexpharm, along with Vingroup and Masan, marked three significant exits by SK Group from Vietnam in 2025.